
Received: AugAccepted: NovemPublished: November 19, 2018Ĭopyright: © 2018 Bonet et al. PLoS Comput Biol 14(11):Įditor: Sarel Jacob Fleishman, Weizmann Institute of Science, ISRAEL (2018) Rosetta FunFolDes – A general framework for the computational design of functional proteins. Overall, FunFolDes provides new means to accomplish the challenging task of functionalizing computationally designed proteins.Ĭitation: Bonet J, Wehrle S, Schriever K, Yang C, Billet A, Sesterhenn F, et al. In particular, the reduced structural compatibility between functional sites and host scaffolds, effectively enabling the repurposing of old protein folds for new functions. These results highlight the capability of FunFolDes to address common challenges on the design of functional proteins.
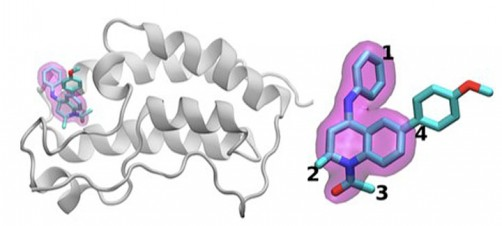
The designed proteins were experimentally characterized, showing that functionalization was successfully achieved. Further, we used FunFolDes to design two novel functional proteins, displaying two viral epitopes that can be of interest for vaccine development. We performed extensive in silico benchmarks, and found that when the design of function is required the global energy minima may not be the optimal solution, in line with previously reported experimental studies.

We developed a computational protocol (Rosetta FunFolDes) to facilitate the insertion of functional motifs into heterologous proteins. Due to our limited understanding of the principles that govern protein function and structure, the computational design of functional proteins remains challenging. The ability to use computational tools to manipulate the structure and function of proteins has the potential to impact many facets of fundamental and translational science. This may lead to important improvements on the computational design of proteins, with structurally complex functional sites, that can perform elaborate biochemical functions related to binding and catalysis. Overall, we present an accessible strategy to repurpose old protein folds for new functions. The designed proteins were experimentally characterized showing high binding affinities to monoclonal antibodies, making them valuable candidates for vaccine design endeavors. To test the design capabilities of FunFolDes we transplanted two viral epitopes into distant structural templates including one de novo “functionless” fold, which represent two typical challenges where the designability problem arises. An observation consistent with several experimental studies that have revealed function-stability tradeoffs. We performed extensive computational benchmarks, where we observed that the enforcement of functional requirements resulted in designs distant from the global energetic minimum of the protein. Here, we present a computational approach that couples conformational folding with sequence design to embed functional motifs into heterologous proteins-Rosetta Functional Folding and Design (FunFolDes). One promising route to enhance the “designability” of protein structures is to allow backbone flexibility. The structural complexity of the functional motif largely determines how readily one can find host protein structures that are “designable”, meaning that are likely to present the functional motif in the desired conformation.
One of the most simplistic approaches for the design of function is to adopt functional motifs in naturally occurring proteins and transplant them to computationally designed proteins. The low success rates of computational design protocols and the extensive in vitro optimization often required, highlight the challenge of designing proteins that perform essential biochemical functions, such as binding or catalysis. The robust computational design of functional proteins has the potential to deeply impact translational research and broaden our understanding of the determinants of protein function and stability.


 0 kommentar(er)
0 kommentar(er)
